The conduct of acidic oxides may be summarised in two broad assertions. When they react with an acid they produce salt and water showing basic properties.

Acidic And Basic Oxides And Hydroxides Youtube
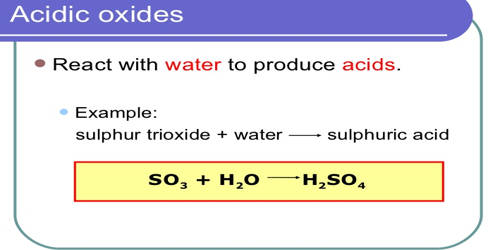
The best examples would be sulfur dioxide sulfur trioxide nitrogen dioxide and carbon dioxide.
. Oxides are characterised as. All these dissolve in water to form respectively sulfurous acid sulfuric acid nitric acid and. M 2 O 3 MO 2 ThO 2.
When nitrogen dioxide reacts with water it gives nitric acid and nitrous acid. Acid oxides are also known as acid anhydrides. The oxides formed by the combination of non metals and oxygen.
Here notrigen dioxide NO2 is the acidic oxide example. The non metals belongs to group 14-17. Acidic oxides are known as acid anhydrides example- sulfur dioxide is sulfurous anhydride and sulfur trioxide is sulfuric anhydride and when combined with bases they produce salts.
Nitrogen dioxide NO 2 sulphur dioxide SO 2 carbon monoxide CO and carbon dioxide CO 2 are some of the oxides found in the air. CO 2gH 2OlH 2CO 3aq Online Classes. Na 2 O H 2 O 2NaOH.
Acid oxides are also known as acid anhydrides. Plenty to go at. The oxides of aluminium and zinc.
Non-metals react with oxygen to form acidic compounds of oxides which are held together by covalent bonds. Amphoteric Oxides in Periodic TableIn the given period the oxides progress from strongly basic through the weakly basic oxides amphoteric and weakly acidic to strongly acidic oxides. The oxides of non-metals are acidic in nature.
When dissolved in water they form acidic solution. Amphoteric oxides have both acidic and basic properties. Examples of acid oxides are sulfurous acid carbonic acid and sulfuric acid.
Cr2O3 H2O H2Cr2O4. The word amphoteric means both of them acid and base. Some examples of this type of oxide are rmNrma_2rmO sodium oxide rmK_2rmO potassium oxide rmMgO magnesium oxide.
Sulphur dioxide SO2 Nitrogen dioxide NO2 Phosphorus pentoxide P4O10 Sulphur trioxide SO3 Carbon dioxide CO2 Chlorine Dioxide ClO2. An oxide which combines with water to form an acid. However while responding with alkalis they form salt and water showing acidic.
SO 3 and dinitrogen pentoxide DNO are examples of oxidants N 2 O 5.

Chemistry Oxides Under Base Alkalis Flashcards Quizlet

What Is The Product Obtained When An Acidic Oxide Reacts With Water Quora
0 Comments